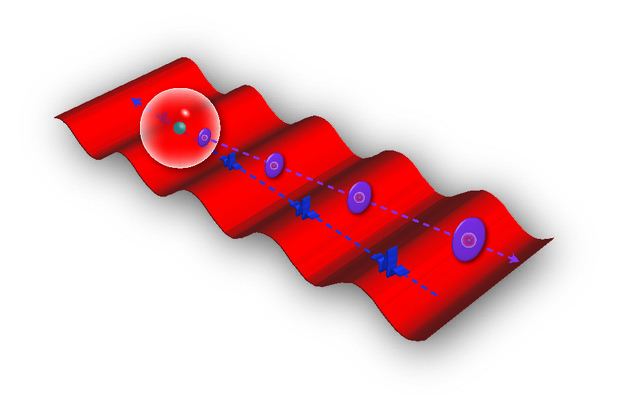
Illustratration the path of packets of light waves (blue dashed line, moving right to left), as they hit an atom (white sphere with green nucleus) and an electron is knocked free (purple dashed line, moving left to right). The red laser light waves below serve as a frame of reference, so researchers can observe the momentum of the electron as it gets farther from the atom. Credit: Courtesy of Robert Jones, University of Virginia
Blink for a second and miss it a quintillion — 1 million raised to the power of five — times. Researchers at Ohio State didn’t miss the moment an electron left an atom as they became the first to observe the phenomenon.
The researchers, led by Louis DiMauro, a physics professor at Ohio State, tracked the departure of the electron as the atom absorbed light.
The discovery will allow for mapping of the tiny universe of atoms on a larger scale and eventually give researchers the ability to manipulate the movement of subatomic particles — pieces of an atom — within a molecule, DiMauro said.
“For instance, how does charge transfer within a molecule? Charge transfer has specific relevance to solar cells because solar cells are nothing more than light hitting some system and the electron becomes excited and generates electricity,” DiMauro said. “What is important if you wanted to make a better solar cell is understanding how the electron did move through the molecular structure of the device.”
The atomic data was gathered through a standard process in quantum physics known as reconstruction of attosecond beating by interfering two-photon transitions. RABITT — a procedure to study processes on extremely small timelines — fires light to hit atoms in gases to reveal information about the subatomic particles inside.
For this project, the team went beyond typical RABITT data and used data that was previously thought to be too complex, DiMauro said. This was the key to observing electrons breaking away from their atoms, he said.
“There was a feeling that it was too complex to disentangle to get the information out of it,” DiMauro said. “I guess there was a general understanding that you would never be able to get it out.”
To crack the complex information, DiMauro and his team put the RABITT data into models and simplified the complexity into a few variables that allowed them to watch the electron leaving the atom, he said.
After witnessing such a moment, Dietrich Kiesewetter, a doctoral candidate in physics at Ohio State, said he sees research possibilities.
“Even more audacious would be to make the same measurements on a changing system (like a molecule in the process of dissociating) taking ‘snapshots’ on the timescale of electronic motion,” he said in an email. “One of the ultimate goals in this field of physics.”
A large part of the study was done in a lab, where many of the observations were done by Kiesewetter, DiMauro said.
“This measurement required a significant amount of time just to accumulate, and because of this long time, also required a high level of stability of our experimental tools (lasers, mostly) and of the environment (building temperature and humidity),” Kiesewetter said. “Each data set that contributed to the published results required about three hours.”


